Generating valuable insight from data inside and outside the organization to create new business value and to optimize processes, is a strategic goal of life sciences organizations across the industry. According to Gartner’s 2018 CIO survey, business and technology leaders in life sciences organizations would like to move beyond rearview analysis into more forward-looking intelligence with advanced capabilities provided by predictive and prescriptive analytics.
When it comes to supply chain, patient engagement, clinical trial patient recruitment and biologic research, we believe investments in advanced analytics offer life sciences organizations the opportunity to compete and operate more effectively, for example:
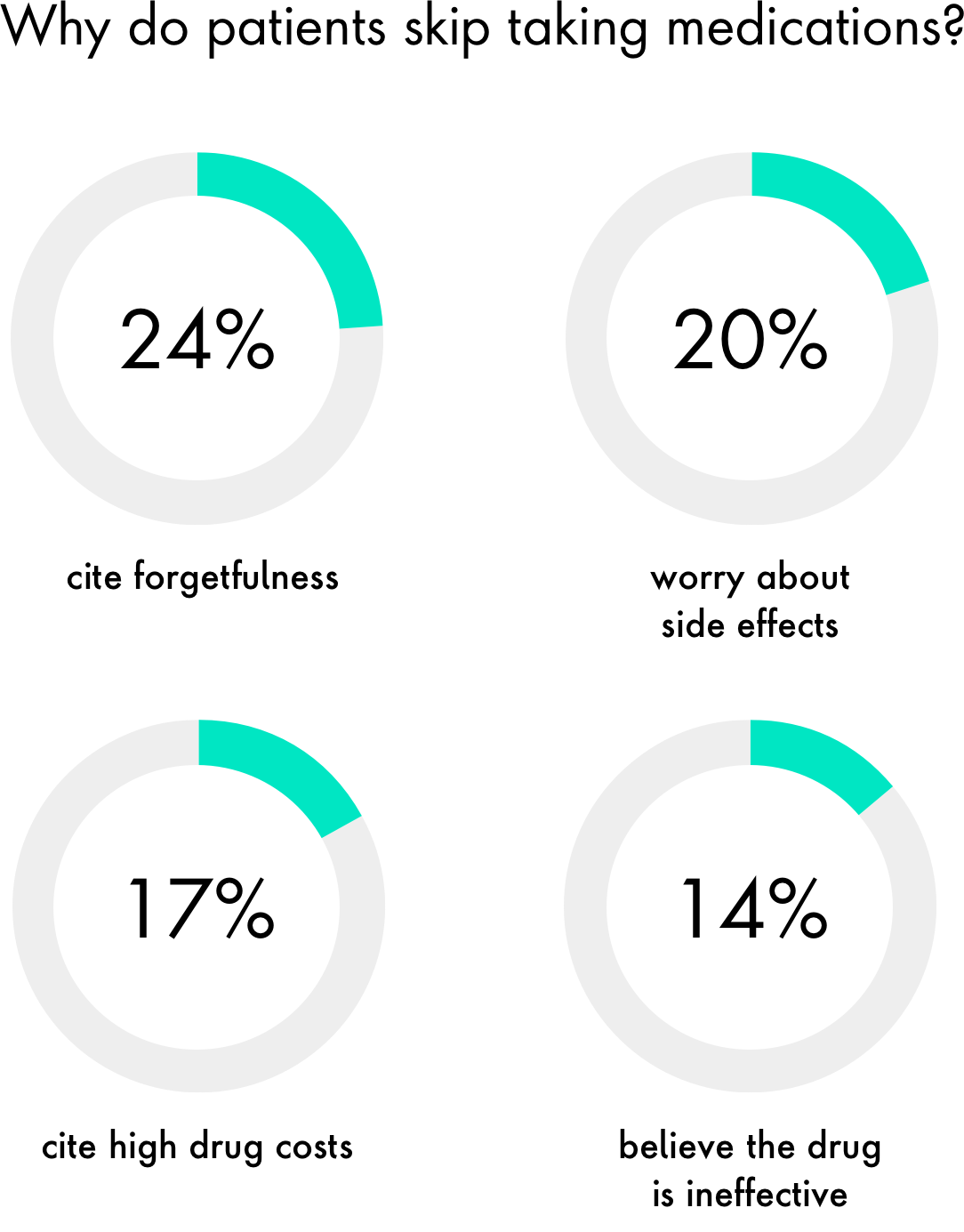
Improve medication adherence
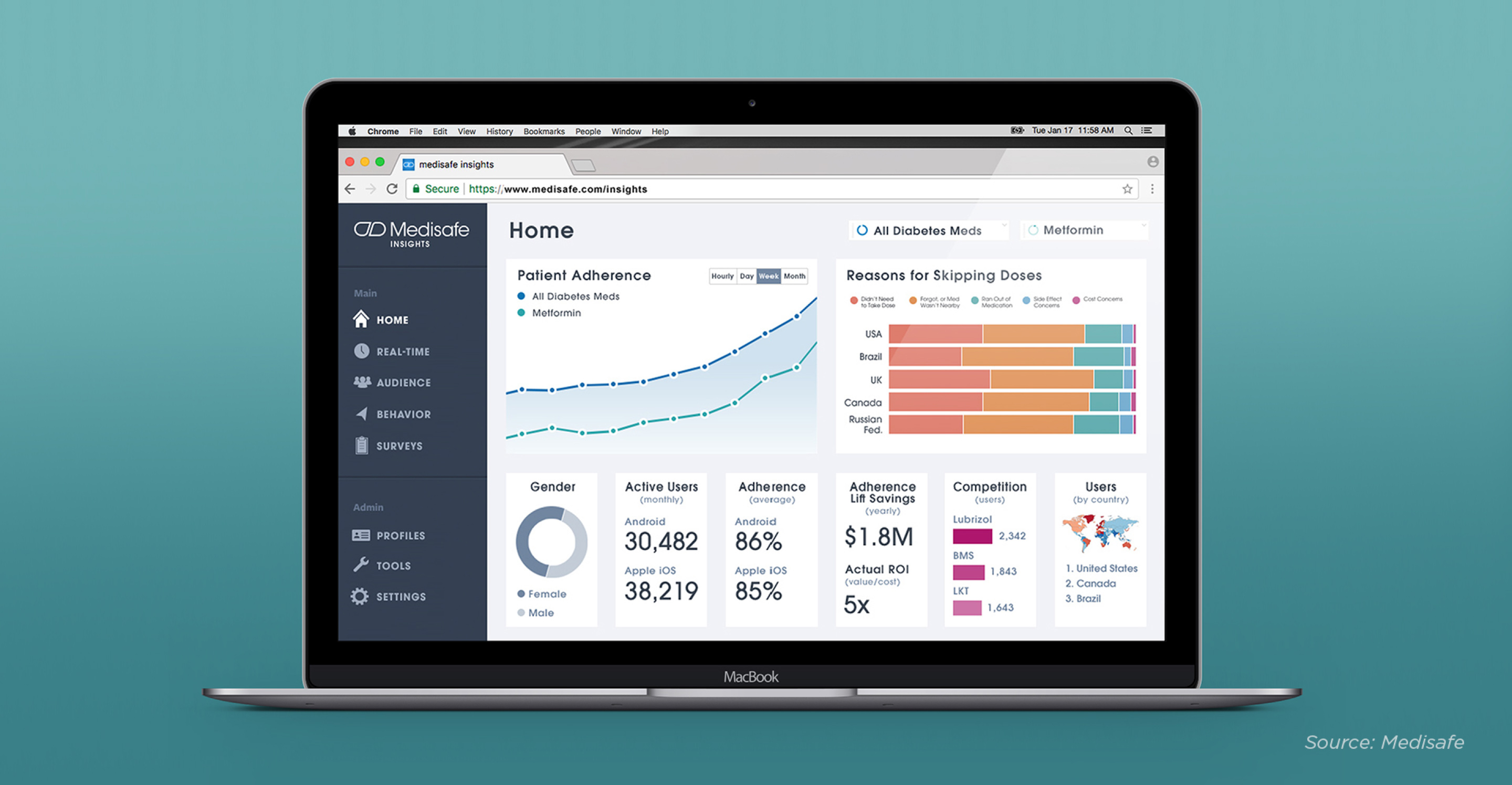
Consider brand-neutral platforms to optimize patient engagement or to increase medication adherence using smartphone apps and Bluetooth-enabled pill bottles. While these inventions are simple in concept, they deliver much-needed innovation to a problem that burdens the healthcare system with billions of dollars in unnecessary costs. Platforms such as Medisafe, have gathered more than 2.5 billion data points that pharma companies can use to gain insight into product and market usage trends.
Find the right opinion leaders
Business leaders in life sciences organizations are starting to do a better job at identifying and segmenting potential key opinion leaders (KOLs) by leveraging big data, advanced analytics and algorithms. By using more advanced techniques, business leaders ultimately engage with the right people to maximize the strength of their organization’s reputation. Graphical tools make it easier to visualize the strength and quality of relevant connections across the community of HCPs.
Recruit qualified patients for clinical studies
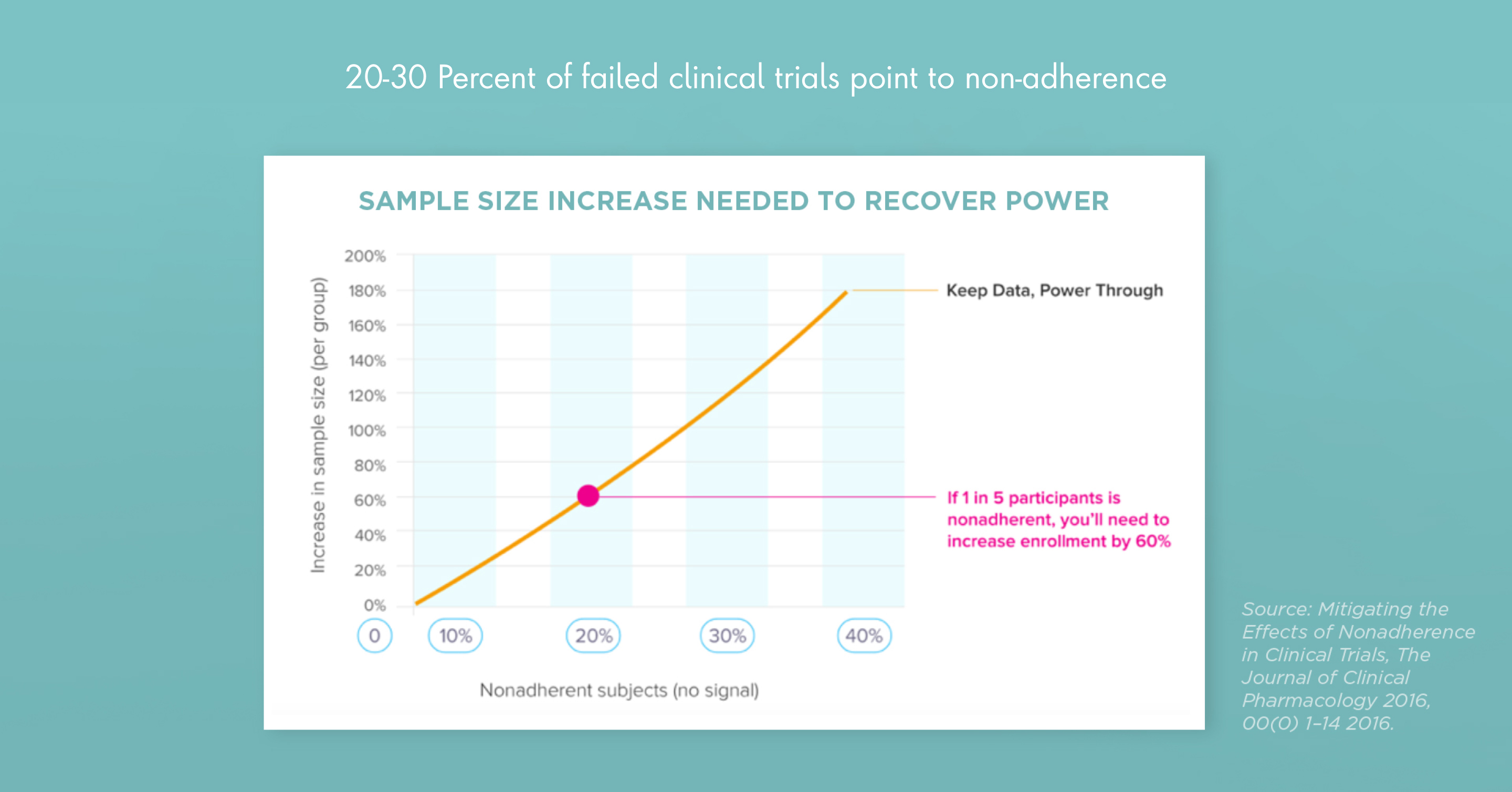
Clinical leaders can improve patient recruitment and adherence, especially in rare disease studies, with modern, innovative techniques for running clinical trials that lead with patient needs. Patient-centric strategies should do everything possible to remove obstacles to trial participation and adherence. Clinical leaders might also consider virtual trials that draw on mobile devices, sensors and wearable technology to make it easy for subjects to participate from home.
Consider lab automation
The market for laboratory automation is more mature than autonomous vehicles or manufacturing/supply chain robotics. New ways to power laboratory instruments, especially in increasingly regulated labs, offer numerous benefits for R&D managers and laboratory IT directors with responsibilities for LotF, laboratory modernization and biologics. Hence, business and technology leaders in life sciences should start to evaluate innovative laboratory automation solutions.
Sources
- 2018 CIO Agenda: Life Science Industry Insights, by Stephen Davies, Gartner; 2 October 2017.
- Demonstrating Double-Digit Impact on Medication Adherence in Hypertension Patients, Medisafe, March 2018.
- Adherence and health care costs, by Aurel Iuga and Maura McGuire, US Library of Medicine, National Institutes of Health, February 2014.
- High Level Expert Forum - How to Feed the World in 2050. Office of the Director, Agricultural Development Economics Division Economic and Social Development Department. Forum held in Viale delle Terme di Caracalla, Rome, Italy